Quantum Numbers Each electron has a set of four numbers, calledquantum numbers, that specify it completely; no two electrons in the same atom can have the same four. That's a more precise statement of the Pauli exclusion principle Bob was discussing. (He also mentioned stillanother way of expressing this important idea.) Each electron has a set of four numbers, calledquantum numbers, that specify it completely; no two electrons in the same atom can have the same four. That's a more precise statement of the Pauli exclusion principle Bob was discussing. (He also mentioned stillanother way of expressing this important idea.)
If "angular momentum" means nothing to you, don't despair. You can also picture its significance this way: l, along with n and the third quantum number, m, is responsible for determining the shape of an electron's probability cloud. Here are a few examples:
|
Monday, October 12, 2009
same set of four quantum numbers
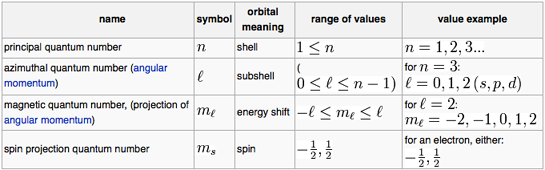
1st - Principle QN n
2nd - Orbital QN l
3rd - Magnetic QN ml
4th - Spin QN ms
n = 1,2,3...7 l goes from 0 to n-1within an energy level
l values = 0 (for s), 1(for p), 2 (for d), 3 (for f) sublevels
Values of ml go from +l to - l , which gives 2l + 1 number of values has 2 values:
+1/2 (spin up) and -1/2(spin down)1. measures the average distanceof the e- from the nucleus 1. indicates the shape of the orbital ( set of probable locations of the e- ) 1. identifies the direction the e-orbital has around the nucleus 1. identifies the "spin" orrotation of the e- about its own axis 2. different values of n mean different energy levels 2. diff. values of l mean diff sublevels. In a sublevel all the e- have nearly the same energy. 2. specifies the e- orbital in which the e- is located within a sublevel. 2. shows that each orbitalcan contain only 2 e- 3. different values of n mean relatively large differences in the energies of the e-s 3. different sublevelswithin the same level may have moderately large differences in energy.
3. different values of ml mean little difference in energies of the e- 3. the direction of spin is either in one direction or the other 4. the smallest avgerage distance and the lowest energy occurs when n = 1; each increase in n increases those quantities. 4. within any level, the lowest energy sublevel is s, then p, then d, then f. 4. the number of possible values of ml within a sublevel idenities how many e- pairs that the sublevel can hold 4. when 2 e- (in an atom) have the same set of QN except for ms, then these e-are called an e- pair 5. the number of e- possible in a level is 2n2 5. the number of possible values of l for a level is equal to the value of n 5.these e- within an e- pair have essentially the same energy
Electron Clouds and Energy Levels Okay, so why is it that s only holds two electrons, but p has room for six? Okay, so why is it that s only holds two electrons, but p has room for six?
|
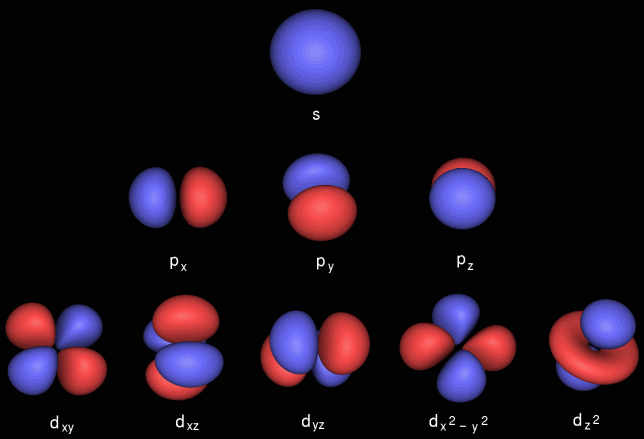
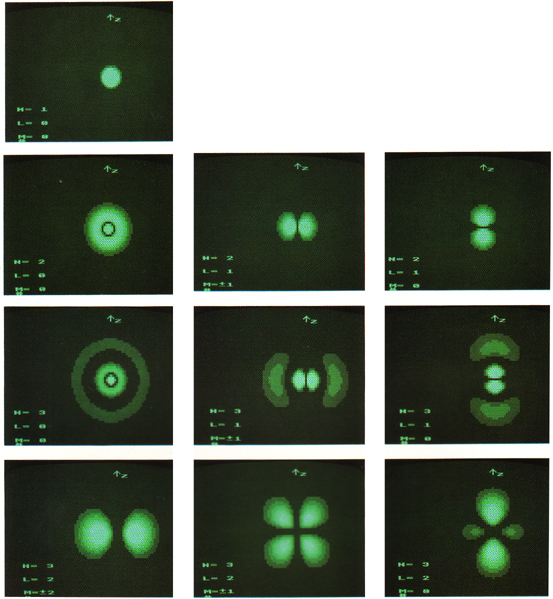
However, p and d states are more interesting: there can be several different-shaped clouds at the same energy. For example, here are two p states from the second primary level:


It turns out that there are three kinds of p clouds in each primary level.
 ...and each one can hold a spin up and a spin down, so that's why six electrons fit into the p column!
...and each one can hold a spin up and a spin down, so that's why six electrons fit into the p column!
 Very good. You can think of each electron's "quantum state," its full, unique description, as being the sum of a particular probability cloud plus a spin:
Very good. You can think of each electron's "quantum state," its full, unique description, as being the sum of a particular probability cloud plus a spin:
 |  | |
 Futhermore, there are five different shapes for d (hence room for ten electrons), and seven in the next sublevel, f...I hope you're noticing a pattern here. (If you want to know more about where these numbers come from, go ask Dr. Mahan about quantum numbers.)
Futhermore, there are five different shapes for d (hence room for ten electrons), and seven in the next sublevel, f...I hope you're noticing a pattern here. (If you want to know more about where these numbers come from, go ask Dr. Mahan about quantum numbers.)
Quantum Numbers to Orbitals
Chemists recognise s, p, d and f-orbitals. The topologies of these orbitals: the shape, phase & electron occupancy are described by four quantum numbers:
n | The principal quantum number |
l | The subsidiary or azimuthal or angular momentum or orbital shape quantum number |
ml | The magnetic quantum number |
ms | The electron spin quantum number |
From Wikipedia:
These quantum numbers conspire to give spherical s-orbitals, dumbbell shaped p-orbitals that come in sets of three, double dumbbell d-orbitals that come in sets of five, etc.:
Electrons enter and fill orbitals according to four rules:
Pauli Exclusion Principal | Orbitals can contain a maximum of two electrons which must be of opposite spin. |
Aufbau or Build-up Principle | Electrons enter and fill lower energy orbitals before higher energy orbitals. |
Hund's Rule | When there there are degenerate (equal energy) orbitals available, electrons will enter the orbitals one-at-a-time to maximise degeneracy, and only when all the orbitals are half filled will pairing-up occur. This is the rule of maximum multiplicity. |
Madelung's Rule | Orbitals fill with electrons as n + l, where n is the principal quantum number and l is the subsidiary quantum number. This rule 'explains' why the 4s orbital has a lower energy than the 3d orbital, and it gives the periodic table its characteristic appearance. |
Certain 'magic' numbers of electrons of electrons exhibit energetic stability: 2, 10, 18, 36, 54, 86 and,one assumes, 118, are associated with the Group 18 inert or noble gases: He, Ne, Ar, Kr, Xe, Rn & Uuo.
- The 'magic' numbers inevitably arise from the underlying quantum mechanics, but as Richard Feynman told us (here): "I think I can safely say that nobody understands quantum mechanics."
We can predict quantum mechanical patterns, but we don't know why we can predict the patterns. We do not understand QM in terms of a deeper theory.
Now available on the web are Richard Feynman's "Messenger Lectures" where he looks at the nature of physical theory and its relationship with mathematics. Highly recommended!
Quantum Patterns
The pattern of orbital structure is very rich and can be mapped onto the two dimensions of paper in many different ways.
Some mappings emphasize how the orbitals are ordered and filled with electrons, others stress how the chemical elements and their orbitals are ordered with respect to atomic number Z. Each tells us something different about atomic orbital structure and/or elemental periodicity.
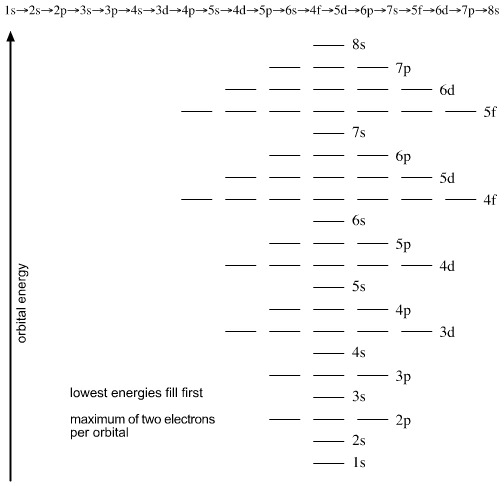
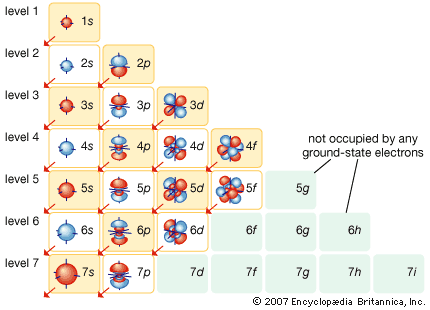
Orbital Filling
The sequence of orbital filling is, from the bottom of this diagram, upwards:
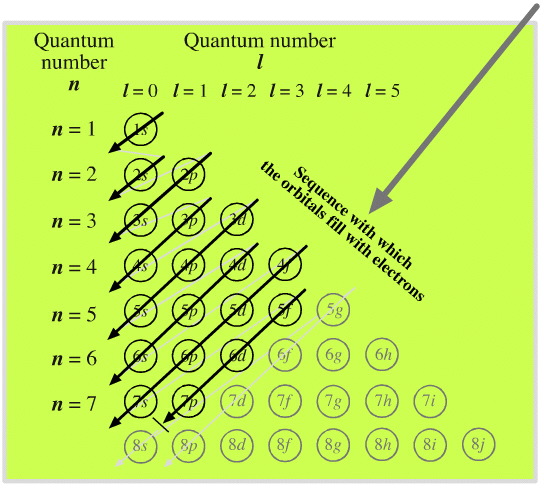
Electron Shells
As electrons are added, the quantum numbers build up the orbitals. Read this diagram, from the top downwards:
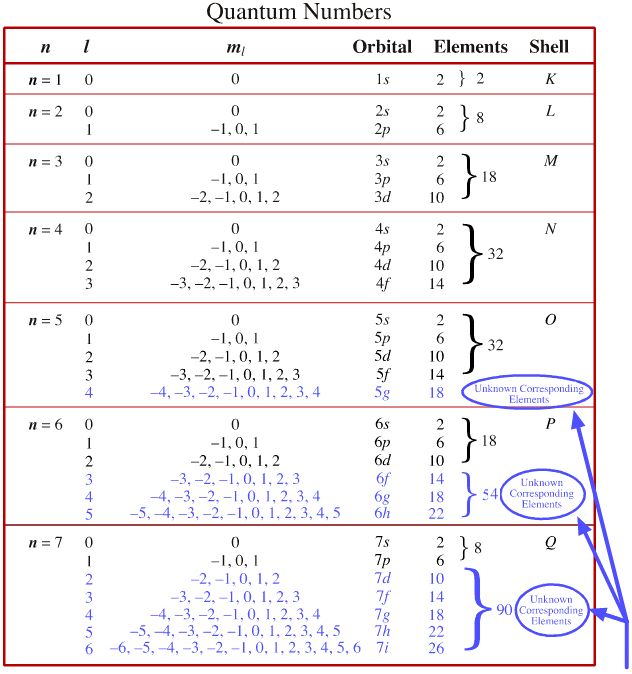
Madelung's Rule says the orbitals fill in the order n + l (lowest first). This gives the sequence:
- (n = 1) + (l = 0) = 1 1s
- (n = 2) + (l = 0) = 2 2s
- (n = 2) + (l = 1) = 3 2p
- (n = 3) + (l = 0) = 3 3s
- (n = 3) + (l = 1) = 4 3p
- (n = 4) + (l = 0) = 4 4s
- (n = 3) + (l = 1) = 4 3d
- (n = 4) + (l = 1) = 5 4p
- (n = 5) + (l = 0) = 5 5s
- and so on...
A nice electron shell representation from the Encyclopedia Britannica:
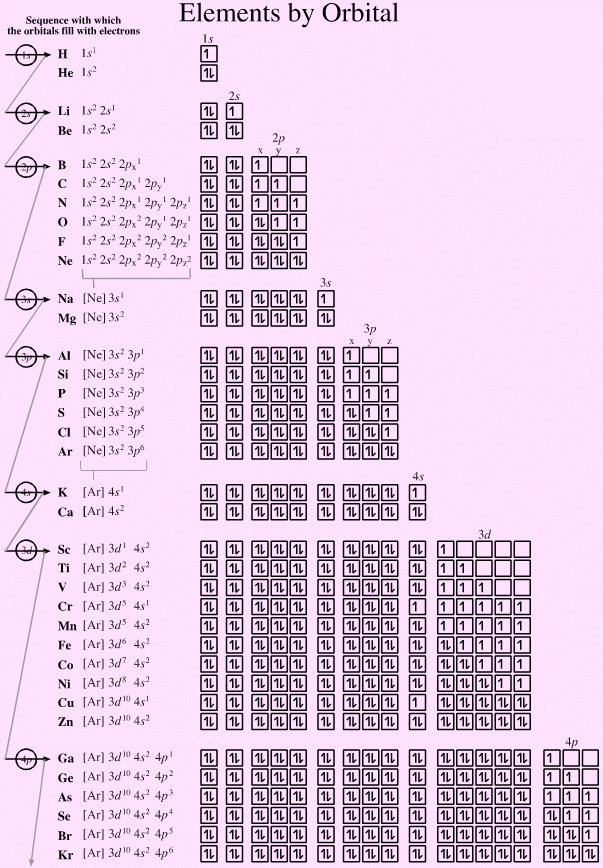
Elements by Orbital, And Some Subtleties...
The electronic structure can be illustrated adding electrons to boxes (to represent orbitals). This representation shows the Pauli exclusion principle, the aufbau principle and Hund's rule in action.
There are some subtle effects with the d block elements chromium, Cr, and copper, Cu. Hund's rule of maximum multiplicity lowers the energy of the 3d orbital below that of the the 4s orbital, due to the stabilisation achieved with a complete and spherically symmetric set of five 3d orbitals containing five or ten electrons. Thus,
- Chromium has the formulation: [Ar] 3d5 4s1 and not: [Ar] 3d4 4s2
- Copper has the formulation: [Ar] 3d10 4s1 and not: [Ar] 3d9 4s2
Introduction
Massive atomic nuclei are born naked, but their net positive charge, Z, quickly attracts Z comparatively mass-less electrons to produce neutral atoms.
- The nucleus of a neon atom – atomic number 10 and so the Ne10+ ion – attracts 10 negatively charged electrons to give an atom of neon.
The negatively charged electrons do not nuclear react with the positively charged protons of the nucleus, as they do inside neutron stars, instead they associate as three dimensional resonant standing waves.
The electrons move about the point positive charge
in a beautiful and subtle quantum mechanical dance
- The modes of resonance for single electron systems such as the hydrogen atom are described by the Schrödinger wave equation.
- Schrödinger knew of de Broglie's proposal that a moving particle has wavelength, l, proportional to Plank's constant, h, and momentum p so that l = h/p, a property we now known as wave-particle duality.
- Schrödinger constructed a differential equation for a wavelike electron resonating in three dimensions about a point positive charge (Wikipedia):
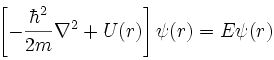
- Solutions to the Schrödinger wave equation correspond to modes of electron resonance and are formally called wavefunctions.
- Wavefunctions assume discrete, or quantised, energies and have energies which correspond to the spectral lines of one electron atoms and ions of the type: H•, He+, Li2+, Be3+, etc.
- Although not exactly the same, chemists tend to call wavefunctions "orbitals".
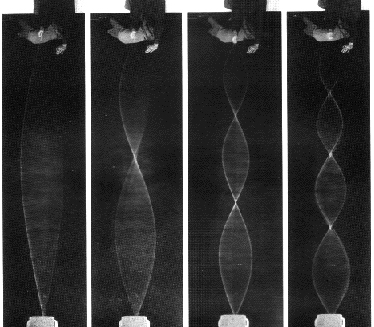
1-Dimensional resonant standing waves, the vibrating string:
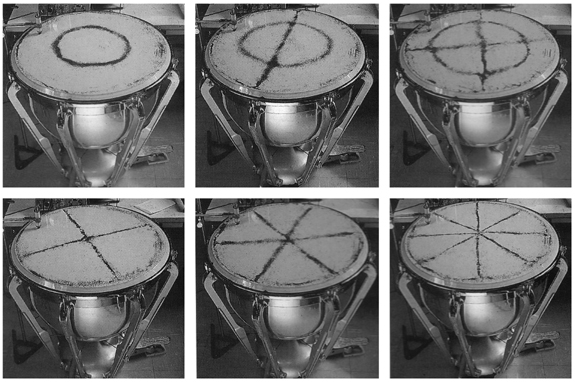
2-Dimensional resonant standing wave, a vibrating drum skin (see QuickTime movies here andhere):
3-Dimensional resonant standing waves, atom wavefunctions from the Schrödinger wave equation:
Electron Clouds and Energy Levels Okay, so why is it that s only holds two electrons, but p has room for six? Okay, so why is it that s only holds two electrons, but p has room for six?
|
However, p and d states are more interesting: there can be several different-shaped clouds at the same energy. For example, here are two p states from the second primary level:


It turns out that there are three kinds of p clouds in each primary level.
 ...and each one can hold a spin up and a spin down, so that's why six electrons fit into the p column!
...and each one can hold a spin up and a spin down, so that's why six electrons fit into the p column!
 Very good. You can think of each electron's "quantum state," its full, unique description, as being the sum of a particular probability cloud plus a spin:
Very good. You can think of each electron's "quantum state," its full, unique description, as being the sum of a particular probability cloud plus a spin:
 |  | |
 Futhermore, there are five different shapes for d (hence room for ten electrons), and seven in the next sublevel, f...I hope you're noticing a pattern here. (If you want to know more about where these numbers come from, go ask Dr. Mahan about quantum numbers.)
Futhermore, there are five different shapes for d (hence room for ten electrons), and seven in the next sublevel, f...I hope you're noticing a pattern here. (If you want to know more about where these numbers come from, go ask Dr. Mahan about quantum numbers.)
   |
Followers
Blog Archive
-
▼
2009
(47)
-
▼
October
(47)
-
▼
Oct 12
(11)
- Quantum NumbersEach electron has a set of four num...
- Quantum NumbersPauli Exclusion Principle: no two e...
- Electron Clouds and Energy LevelsOkay, so why is i...
- Quantum Numbers to OrbitalsChemists recognise s, p...
- The electronic structure atoms can be understood i...
- The Electronic Structure of Atoms
- Electron Clouds and Energy LevelsOkay, so why is i...
- Rules for Electron ConfigurationsI wish you'd stop...
- Electron Configurations ContinuedTake a look at th...
- SpinSpin? What's that? Well, it's an additional pr...
- The Pauli Exclusion PrincipleSee what happens when...
-
▼
Oct 12
(11)
-
▼
October
(47)
 Is there a special reason why there are four, and not three or six or fifty-nine?
Is there a special reason why there are four, and not three or six or fifty-nine?  Good question. There are certainly reasons, but I won't be able to explain them to you here, any more than Bob could explain where his rules were coming from. What I can offer you is a mathematical expression of those rules, which I hope will make them easier to work with and perhaps provide some insight into the underlying patterns.
Good question. There are certainly reasons, but I won't be able to explain them to you here, any more than Bob could explain where his rules were coming from. What I can offer you is a mathematical expression of those rules, which I hope will make them easier to work with and perhaps provide some insight into the underlying patterns.  Okay, I can live with that. Tell me about the four numbers.
Okay, I can live with that. Tell me about the four numbers.  First, the "primary quantum number," which is given the symbol
First, the "primary quantum number," which is given the symbol  All right, so n tells you which of the "main" energy levels you're in. I suppose there's another quantum number that goes with the sublevels--
All right, so n tells you which of the "main" energy levels you're in. I suppose there's another quantum number that goes with the sublevels-- Very good. The second quantum number is known as l. A value of l=0 corresponds to s, l=1 is p, l=2 is d, and so forth.
Very good. The second quantum number is known as l. A value of l=0 corresponds to s, l=1 is p, l=2 is d, and so forth.  This all seems very abstract to me. What does l reallymean? Can you give me some concrete way to think about it?
This all seems very abstract to me. What does l reallymean? Can you give me some concrete way to think about it?  I have two answers for that. First, l, unliken, does have an association with angular momentum. If you'd like to know more about this, click on the "advanced" button at right.
I have two answers for that. First, l, unliken, does have an association with angular momentum. If you'd like to know more about this, click on the "advanced" button at right.





 So you're saying that p electrons have more cloud shapes available to them?
So you're saying that p electrons have more cloud shapes available to them?